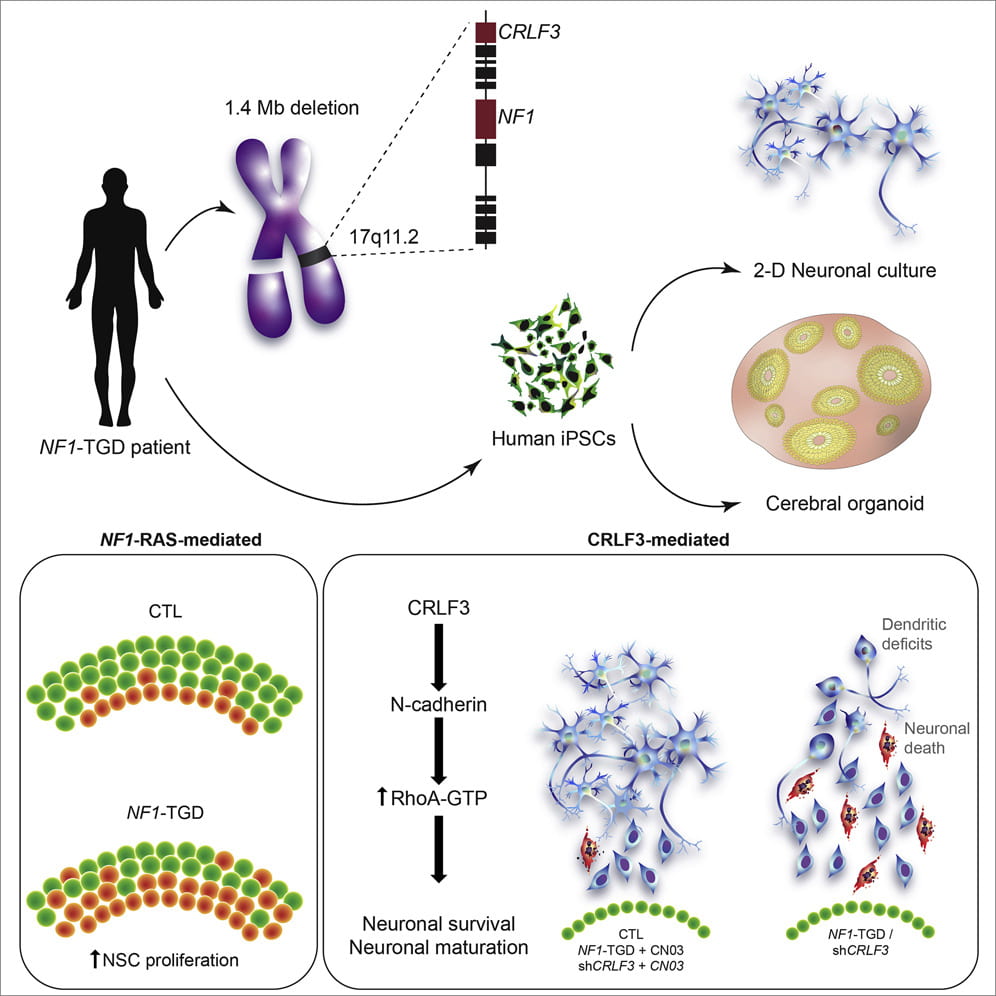
Neurodevelopmental disorders are often caused by losses of large pieces of chromosomes containing many genes. This is also true for a subset of individuals with Neurofibromatosis type 1 (NF1) who have severe developmental delays and intellectual disabilities. These NF1 patients often harbor a large deletion involving the NF1 gene on chromosome 17q11.2, termed a total gene deletion (NF1-TGD).
To understand why children with this chromosomal deletion have such profound neurological deficits, Dr. Michelle Wegscheid, a former MD-PhD student in the laboratory of Dr. David Gutmann, teamed up with her colleagues in the NF Center to use patient-derived human induced pluripotent stem cells (hiPSCs) to generate cerebral organoids (hCOs) or “mini-brains”.
In a new report recently published in the journal Cell Reports, Dr. Wegscheid, along with Dr. Corina Anastasaki, Kelly Hartigan, Olivia Cobb, Jennifer Traber, and Dr. Stephanie Morris, identified both neural stem cell growth and neuronal maturation abnormalities in NF1-TGD hCOs. While the increased NSC proliferation resulted from decreased NF1/RAS regulation, they showed that the neuronal differentiation, survival and maturation defects were caused by reduced expression of a gene called cytokine receptor-like factor 3 (CRLF3).
Importantly, they demonstrated a higher autism burden in NF1 patients harboring a mutation in the CRLF3 gene, thus establishing CRLF3 as a new causative gene within the NF1-TGD locus responsible for hCO brain abnormalities and autism in children with NF1.

Michelle Wegscheid, PhD 
Corina Anastasaki, PhD 
Kelly Hartigan, BS 
Olivia Cobb, MS 
Jason Papke, MS
Wegscheid ML, Anastasaki C, Hartigan KA, Cobb OM, Papke JB, Traber JN, Morris SM, Gutmann DH. Patient-derived iPSC-cerebral organoid modeling of the 17a11.2 microdeletion syndrome establishes CRLF3 as a critical regulator of neurogenesis. Cell Rep 2021; July 6 36:109315. doi: 10.1016/j.celrep.2021.109315 PMID:34233200.