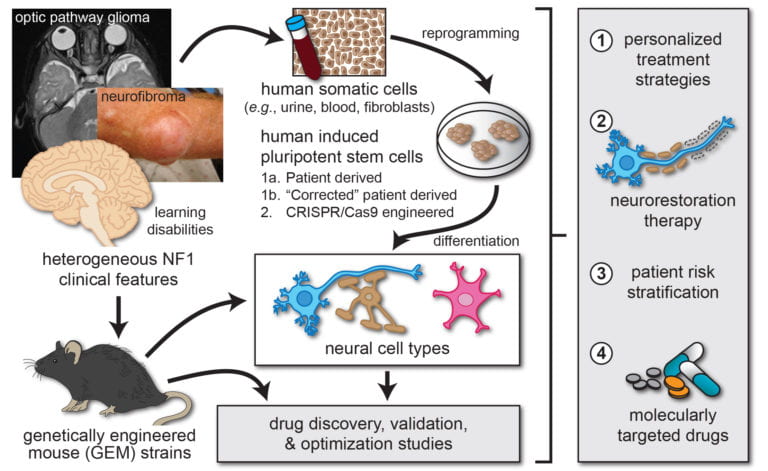
A critical first step towards identifying new treatments for people affected with brain tumors and cognitive delays is the development of accurate preclinical models of human disease. To this end, we have leveraged genetic and cellular recombineering technologies to generate both genetically engineered mice and human induced pluripotent stem cell (iPSC) models to study NF1 disease pathogenesis, as well as to discover and evaluate promising therapies.
Genetically engineered mice
Over the past 20 years, our laboratory has generated numerous genetically engineered mouse models of NF1-associated brain tumors, NF1-associated attention and learning deficits, and sporadic pediatric low-grade brain tumors. We have extensively credentialed these models and used them to identify several new promising treatments for NF1-associated learning deficits and optic glioma, which have entered clinical trials for children with NF1. In addition, these models provide highly instructive platforms to critically dissect the pathogenesis of human disease.
Current projects in the Gutmann research laboratory are focused on developing and employing mouse models harboring patient specific germline NF1 gene mutations for mechanistic discovery projects and preclinical studies testing promising therapeutic compounds.
Human Induced Pluripotent Stem Cells
Over the past 12 years, our laboratory has established a rich repository of human induced pluripotent stem cell lines, each harboring different NF1 gene mutations. This invaluable resource has proved invaluable to study the effect of NF1 gene mutation on human nervous system cell function.
Current projects are leveraging these cells as sources for cerebral organoid cultures (“mini brains”) and differentiated progenitor cells to study normal brain development, to create human cellular models of low-grade and malignant brain tumors, and as platforms to examine the contribution of germline genetics and sex to human microglia and neuron function.