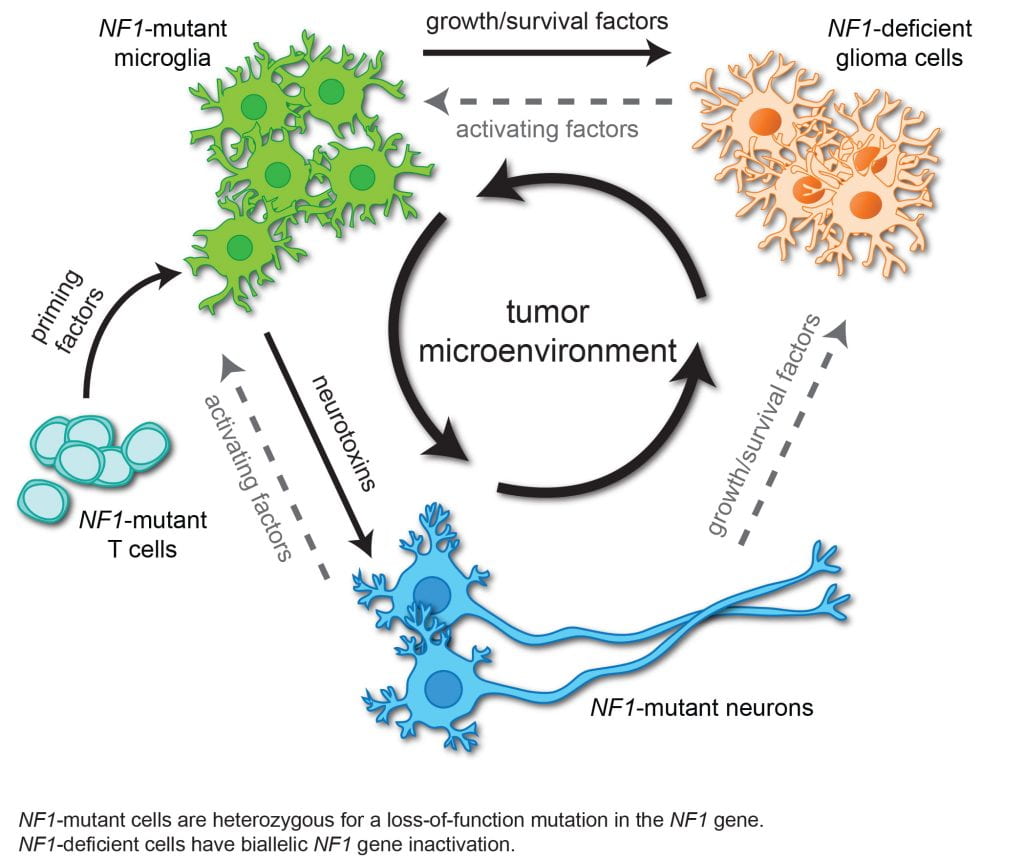
Brain tumors are heterogeneous cancers in which 50% of the cells in the tumor are non-neoplastic (stromal) cells. Over the past decade, we have employed a variety of genetic and pharmacological methods to firmly establish that immune cell function is critical for low-grade glioma formation and growth in mice. Leveraging single cell RNA-sequencing methods and novel bioinformatic approaches, we have begun to identify the stromal signals that dictate glioma growth in vivo.
To this end, we have shown that CD8+ T cells are required for pediatric low-grade glioma growth, such that inhibiting their infiltration into the tumor, blocking their function, or attenuating their activation by neurons abrogates tumor progression. Moreover, T cells also serve as sentinels of systemic disease. As such, T lymphocytes responding to asthma or gut microbial colonization block optic glioma growth.
Current projects in the Gutmann research laboratory are focused on understanding the role of T cells in maintaining both NF1-associated and sporadic pediatric low-grade glioma growth, defining the role of T cells as systemic sensors of disease (risk factor mediators), applying bioinformatic approaches to characterize the low-grade glioma ecosystem in human and mouse tumors, and creating new reagents to investigate the contribution of T cells to neuronal function in health and disease.